Dear readers, I am here with another exciting n’ informative article about ‘Physical and Chemical Changes’. In this post, we have revealed all those contents and common questions that click your mind while studying this topic, as mentioned below in the table of contents. Please read the article to get substantial knowledge about the topic. THANK YOU!
Introduction
There are different types of changes that occur in our everyday life. Changes: for example- change of weather, change of seasons, and occurrence of natural calamities, tooth decay, (growth of man, plants and civilizations), change of state of water and many more. Changes can be physical or chemical. Let us have a brief discussion on the topic through a mind map (that will enlighten you with the concepts of this article).
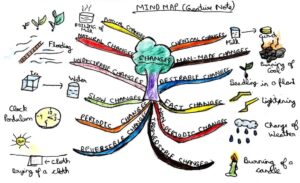
As and when you examine these types of changes, you notice that all these are interconnected. It means that if a change falls under a category of periodic change, it may fall under the category of desirable and slow change too.
For example – ‘rusting of iron’ is an undesirable, non-periodic, slow, natural, irreversible and chemical change. So, we are now going to have a universal view of these points.
DESIRABLE & UNDESIRABLE CHANGES
Desirable changes
They are the changes that produce good and useful results (of your choice/desire; favourable). For example: curdling of milk, germination of seeds, seeding in plants, ripening of fruits, crops getting water through rain.
Undesirable changes
They are the changes that produce bad or useless results (not of your choice/desire; unfavourable). For example: flooding that destructs, ozone holes, drought, rotting of fruits, cutting of trees, milk that turns sour on a blistering day.

PERIODIC & NON-PERIODIC CHANGES
Periodic changes
They are the changes that occur at regular intervals (periodically-consecutively-again and again). For example: change of seasons, movement of clock pendulum, occurrence of day and night, change of moon phases, change of heart-beat and pulse rate.
Non-periodic changes
They are the changes that can occur at any point of time (unpredictable). For example: burning of matchstick, natural calamities (flood, cyclone, earthquake and volcanic eruptions), thunder and lightning, rusting of iron, cutting of trees, growth in humans and plants.

SLOW & FAST CHANGES
Slow changes
They are the changes that occur leisurely (slowly) and take days, months or years to accomplish. For example: growth of man and plants, rusting of iron, tooth decay, change of seasons.
Fast changes
They are the changes that occur very rapidly (fast) just in seconds. For example: burning of matchstick, lightning, switching on/off a bulb, burning of crackers.

NATURAL & MAN-MADE CHANGES
Natural changes
They are the changes that occur by the influence of nature. For example: growth of man and plants, rusting of iron, change of seasons, natural calamities.
Man-made changes
They are the changes that occur by the influence of man. For example: burning of non-renewable energy resources (coal and petroleum), conversion of iron to steel, cutting of hair.

REVERSIBLE & IRREVERSIBLE CHANGES
Reversible changes
They are temporary changes that can recover back into the original form. For example: change of state of water (water cycle), change of seasons, switching on/off a bulb.
Irreversible changes
They are permanent changes that cannot recover back into the original form. For example: curdling of milk, rusting of iron, burning of magnesium, burning of paper.

PHYSICAL & CHEMICAL CHANGES
Physical Changes
They are temporary and reversible changes in which ‘no new substance is formed’. For example: magnetizing a piece of iron, drying of clothes, separating iron from iron sulphur mixture, change of state of water, drying of a fruit.
When a physical change occurs, the substance changes in shape, size, appearance or state; but never changes its chemical composition. E.g. change of state of water (water → ice). Even if water (as a medium) is heated or cooled to form water vapour and ice, they remain chemically ‘H2O’. The only difference is the change of temperature or pressure of water to change the state of water. That means, the properties of the original substance are not altered (changed).
Chemical Changes
They are permanent and irreversible changes in which ‘new substances form’. For example: respiration, fermentation, boiling of eggs, forming compound by heating iron sulphur mixture, burning of matchstick, rotting of eggs, ripening of fruit.
When a chemical change occurs, the properties and the chemical composition of the substance differ. E.g. when milk changes to curd (cannot be reversed and the chemical composition of milk changes).

[wptb id=359]
Question: What is SUBLIMATION?
Answer: The process by which a solid directly changes to gaseous form, without forming liquid, is sublimation. For example: camphor, naphthalene balls, dry ice and iodine.

INVOLVEMENT OF ENERGY
- Physical change – use energy to change state of matter.
- Chemical change – release or absorb energy when changing an original substance to a new substance.
Release energy: during decomposition (breakdown) of a substance.
Absorb energy: during formation of a new substance.
EXTRA QUESTIONS
Q1. What happens when SOLID IODINE is heated?
When we heat solid iodine, it converts directly to gaseous iodine because it sublimes.
Q2. Why heating a platinum wire is a physical change but burning of magnesium wires a chemical change?
Heating of a platinum (Pt) wire is a temporary, reversible and no new substances are formed (Pt is a noble metal), whereas Mg wire on burning forms a new substance MgO (white ash). The change is permanent and irreversible.
Q3. Why RAIN CYCLE is a physical change?
Rain cycle is a physical change because water only changes its state and goes round and round without forming any other substances. Water does not change its chemical composition and remains H2O.It is a temporary and reversible process.
Q4. Why is ACID RAIN a chemical change?
Acid rain is a chemical change because in industrial regions, when rain occurs, it mixes with sulphuric acid and nitric acid and forms new substances. It is permanent and irreversible.
Q5. What happens when iron is exposed to air and moisture?
When iron is exposed to air and moisture, iron will get rusted (chemical change).
Q6. What happens when a magnet is brought near to iron sulphur mixture (powder)?
When a magnet is brought near to iron and sulphur powder, the iron particles in the mixture will stick to the magnet. As a result, iron will get separated from the powder.
Q7. Rohan has a mixture of Iodine and Table salt. He has to separate the mixture by physical method. What should he do?
Rohan will use the method of Sublimation. As Iodine is sublimable it will form I2 vapours. Non-sublimable Table salt will remain behind.
Q8. How is DRYING OF A FRUIT a physical change?
Drying of a fruit is a physical change because only the water evaporates and the chemical composition of the fruit remains the same.
Q9. How BURNING OF COAL is a chemical change?
When coal burns, carbon dioxide and ash are the new products. The change is not reversible by cooling the new products, thus it is a permanent and irreversible change.
Q10. How HEATING SUGAR is a chemical change?
When we heat sugar in a test tube, sugar melts, droplets of water appear on the cooler parts of the apparatus and leaves behind new products, i.e. black carbon and water. The change is not reversible and we cannot obtain original products by reacting the new products, thus it is a permanent and irreversible change.
Q11. How PHOTOSYNTHESIS is a chemical change?
Photosynthesis is a process that involves the combination of carbon dioxide and water in the presence of sunlight and chlorophyll to produce glucose and oxygen. We cannot obtain carbon dioxide and water back form glucose and oxygen, thus it is a permanent and irreversible change.
Star Fact
NH4Cl (Ammonium Chloride – solid) is a unique substance that involves both physical and chemical change. In such a case, when we heat NH4Cl, it sublimes and directly changes to vapour and forms [NH3 + HCl] gas. On cooling, the new products recombine to give back the original substance, i.e. NH4Cl.
Dear readers, we hope that this article is very useful and fruitful to you. If you further have any queries, you can drop in the comment section. You may write your valuable feedback for the article prepared. THANK YOU!!
To get NCERT Chapter 5 Physical and Chemical Changes PDF, click here.
